By Georgina Barone, DVM, DACVIM (Neurology)
Canine congenital encephalopathies comprise a broad range of developmental disorders. Clinical signs are dependent upon the area of the brain affected and may indicate whether the patient has focal, multifocal, or diffuse disease. A thorough neurologic examination is essential to determine neuroanatomic localization and make appropriate diagnostic and treatment recommendations. It is also imperative that other diseases that may contribute to neurologic deficits be ruled out such as hypoglycemia, porto-systemic shunts, infectious diseases, and inborn errors of metabolism. When evaluating a dog with a suspected intra-cranial developmental anomaly, the veterinarian should consider the following factors. First, is the abnormality of clinical significance? Some anomalies do not produce clinical signs and must be interpreted in light of the patient’s condition. Second, the animal must be evaluated for other malformations. Embryologic development of the brain is closely related to that of the spine and other tissues. Critical evaluation of all organ systems is essential to determine the viability of the dog as a pet. Heritability of the problem is also of key importance to the breeder, although many anomalies occur as sporadic occurrences. Lastly, treatment options and quality of life concerns must be addressed as many anomalous conditions of the CNS carry a guarded to poor prognosis with them.
This article will provide information on some of the more common congenital anomalies seen in our canine patients. Acquired disorders will be addressed in a future newsletter.
Quadrigeminal cysts (QC) are widely believed to be developmental anomalies that arise in close proximity to an intra-cranial arachnoid cistern, most commonly the quadrigeminal cistern located above the cerebellum. They represent accumulation of cerebrospinal fluid between sheets of the arachnoid layer of the meninges and lack an epithelial lining, thus are considered “pseudo-cysts”. Once thought to be rare, these anomalies are increasingly being recognized, most likely due to the greater availability of advanced imaging such as CT and MRI.
Small-breed dogs, particularly brachycephalic breeds (especially Shih-tzu’s) and male dogs are over-represented. Although clinical signs can be variable, one of the most common signs are generalized seizures, likely due to the pressure on the occipital lobes of the cerebrum by pressure from the cystic mass. Additionally, ataxia, intention tremors, paresis, and head tilt have all been reported. Support for the developmental nature of this disease stems from the fact that most patients are young (<1 year) of age when diagnosed and histopathology fails to reveal evidence of concurrent scarring, hemorrhage, infection, or inflammation. Occasionally, QC’s are found as incidental findings, either on necropsy or brain imaging.
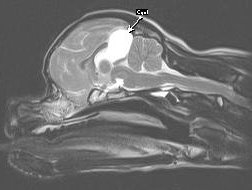 Figure 1 – Arachnoid Cyst
Figure 1 – Arachnoid Cyst
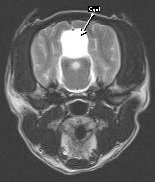 Figure 2 – Arachnoid Cyst
Figure 2 – Arachnoid Cyst
Diagnosis of arachnoid cysts requires advanced imaging, preferably MRI or 3-D CT scanning. MRI (see Figures 1 & 2) will reveal a mass lesion rostro-dorsal to the cerebellum and caudal to the occipital lobes. The lesion appears hyper-intense on T2 images and hypointense on T1 images and is non-contrast enhancing. The quality of the lesion is virtually indistinguishable from cerebrospinal fluid. Variable degrees of compression and distortion of the adjacent cerebellum and cerebrum can be observed. Concurrent hydrocephalus has been reported, but this is likely a breed-related variant of ventricle size and unlikely to be of clinical significance. QC must be differentiated from cystic neoplasia or cysts associated with infectious disease (e.g. hydatid cyst).
Treatment of QC’s include surgery (fenestration, shunting, or marsupialization) or medical management (e.g. corticosteroids and carbonic anhydrase inhibitors), but controversy still remains over the preferred treatment. Prognosis must be considered guarded and the majority of dogs require life-long anticonvulsant therapy, even if surgical correction is performed.
Hydrocephalus refers to an increased volume of cerebrospinal fluid within the ventricular system and is most commonly recognized within the first few months of a dog’s life. The pathophysiology of congenital hydrocephalus is complex and mutifactorial but may be associated with fusion of the rostral colliculi, pre-natal infections causing stenosis of the mesencephalic aqueduct, compromise of cerebral vasculature, and intrauterine toxicity. Breeds at risk include Chihuahua’s, Yorkshire Terrier’s, Poodle’s, and a variety of brachycephalic breeds. Clinical signs generally are apparent prior to 6 months of age and are highly variable but usually include evidence of a prosencephalic disturbance. Abnormal mentation, visual deficits, circling, poor response to training, head pressing, seizures, and pacing have all been reported. Occasionally, hindbrain signs will predominate with ataxia, head tilt, abnormal nystagmus, and balance loss. Physical examination may reveal a large, dome-shaped head (Figure 3), calvarial defects, or an open fontanelle. Bilateral ventrolateral strabismus (“sunset eyes”) is seen as a sequelae to the skull malformation rather than as an indication of a vestibular disturbance.
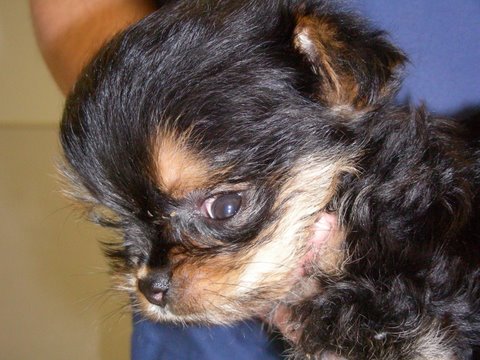
Figure 3 – Dome-shaped head in Hydrocephalus
Diagnosis is generally straightforward and is based on signalment, clinical signs, physical exam findings, and confirmation of ventriculomegaly. It must be understood that enlargement of the ventricles and the presence of an open fontanelle are not necessarily of any clinical significance. The patient must demonstrate signs of a brain disorder in the absence of any concurrent, active causal disease that may be responsible (e.g. encephalitis, metabolic encephalopathy). In patients with a patent fontanelle, the diagnosis may be confirmed with ultrasonography. Advanced imaging (CT, MRI) is the preferred method for imaging and confirming the diagnosis and for ruling out any concurrent disorders. MRI (Figure 4) will reveal dilation in the ventricular system and loss of the adjacent parenchyma.
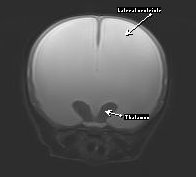
Figure 4 – Hydrocephalus MRI
Treatment is aimed at reducing CSF volume and production. Prednisone or carbonic anhydrase inhibitors have been used with variable success. Surgical intervention is the treatment of choice and is aimed to divert CSF away from the ventricular system to the peritoneal cavity, pleural space, or right atrium. Ventriculoperitoneal shunting is the most common, technically feasible procedure done in domestic animals and can be done even on very small patients, such a Chihuahua’s. Prognosis is highly variable and depends on the degree of pre-operative neurologic dysfunction, chronicity, and avoidance of complications (infection, occlusion) associated with the shunt. Success rates as high as 90% have been reported with ventriculoperitoneal shunts, but owners must be advised that long-term prognosis for full return to function is guarded.
Chiari-Like Malformation (Caudal Occipital Malformation Syndrome or “COMS”) is a developmental anomaly that is being increasingly recognized as advances in neuroradiology are made. Anatomic abnormalities of the skull, specifically the occiput, result in compression of the structures of the caudal fossa and lead to cerebellar herniation. Consequently, there is alteration in the dynamics of cerebrospinal fluid flow and pressure on the cranial aspects of the spinal cord. Pressure gradients resulting from altered CSF flow as well as constriction of the cervicomeduulary junction at the foramen magnum result in the development of excessive fluid buildup within the spinal cord, either confined to the central canal (hydromyelia) or within the neuroparenchyma (syringomyelia). Collectively, the condition is referred to as syringohydromyelia (SM).
COMS is most often diagnosed in small breed dogs, especially Cavalier King Charles Spaniels. Other breeds being seen with increasing regularity include the Pomeranian, Pug, and other brachycephalic breeds. Affected animals can display a wide array of clinical signs including cerebellovestibular dysfunction, seizure activity, cervical/cranial hyperesthesia, or persistent scratching at the neck and shoulder region. Although it is considered to be a developmental disorder (most are diagnosed by 3 years), age at diagnosis can vary and clinical signs may not be evident until the animal is several years old. This is likely due to the fact that syringomyelia may take years to develop; the author has observed dogs that did not begin to display clinical signs until 7 or 8 years of age.
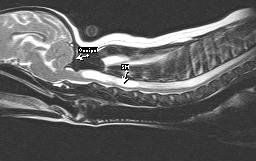 Figure 5 – MRI of COMS & SM
Figure 5 – MRI of COMS & SM
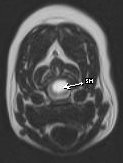 Figure 6 – MRI of COMS & SM
Figure 6 – MRI of COMS & SM
MRI is considered to be the diagnostic tool of choice to confirm COMS and SM (Figures 5 & 6). MRI findings in this condition include rostral displacement of the cerebellum by the occiput, obliteration of the dorsal subarachnoid space at the cervicomedullary junction, and cervical syringohydromyelia. Ventriculomegaly can also be observed but may be a normal variant in brachycephalic breeds and must be interpreted with caution. Additional diagnostics are currently being evaluated by researchers and include spiral CT scanning and brain-stem auditory evoked responses (BAER).
Medical management is directed toward relieving pain and decreasing CSF production. Commonly prescribed drugs include corticosteroids, narcotics, gabapentin, pregabalin, and carbonic anhydrase inhibitors. While medical therapy may effectively alleviate discomfort, long term prognosis is poor if the underlying anomaly is not addressed and treated. Progression of SM leads to pressure on the spinal cord parenchyma leading to permanent nerve damage and eventually intractable pain and paralysis. The treatment of choice in humans with COMS is Foramen Magnum Decompression (FMD) and the majority of human patients that undergo FMD either experience a halt in the progression or improvement in clinical signs. There is increasing evidence that FMD is the preferred method of treatment in dogs as well. Without surgery, more than 1/3 of dogs will be euthanized due to chronic severe pain and quality of life concerns. FMD allows for removal of hyperplastic occipital bone and relieves pressure on the underlying parenchyma. Often, SM will resolve or improve after the procedure, as evidenced by serial MRI exams. Unfortunately, in humans and animals, recurrence rate is high due to formation of scar tissue at the previous surgical site which in essence, recreates the original defect. A modification of the FMD in which titanium is placed over the defect created by the FMD to prevent excessive scar tissue has shown great promise with significantly fewer animals requiring re-operative procedures.
Other brain anomalies: Many other anomalies of the brain have been reported sporadically in dogs and should be considered when evaluating a pediatric patient with intracranial signs. Hydranencephaly has been reported in Labrador Retriever puppies and results from in utero destruction of previously viable neocortex during a critical period of development. Unlike hydrocephalus, the cranial cavity is of normal configuration. Imaging findings are similar to those seen with hydrocephalus but prognosis is extremely guarded. Lissencephaly (Figure 7) occurs when the normal cerebrocortical folds fail to develop, leading to an absence of gyri and sulci of the cerebral hemispheres. Lhasa Apso’s are most commonly affected, but the disease has also been reported in Irish Setters and Wire Hair Fox Terriers. Clinical signs include aggression, blindness, poor training ability, and generalized seizures. Seizures often do not occur until the animal is greater than 1 year of age and tend to be refractory to standard anticonvulsants. Prognosis is grave. Other defects in neuroparenchymal development (Figure 8 ) are seen infrequently and are poorly understood.
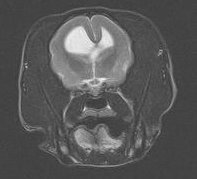 Figure 7 – Lissencephaly
Figure 7 – Lissencephaly
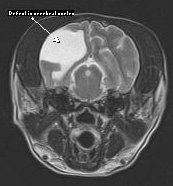 Figure 8 – Defect in neuroparenchymal development
Figure 8 – Defect in neuroparenchymal development







